Ghana FDA
Reimagining Digital Engagement for the FDA
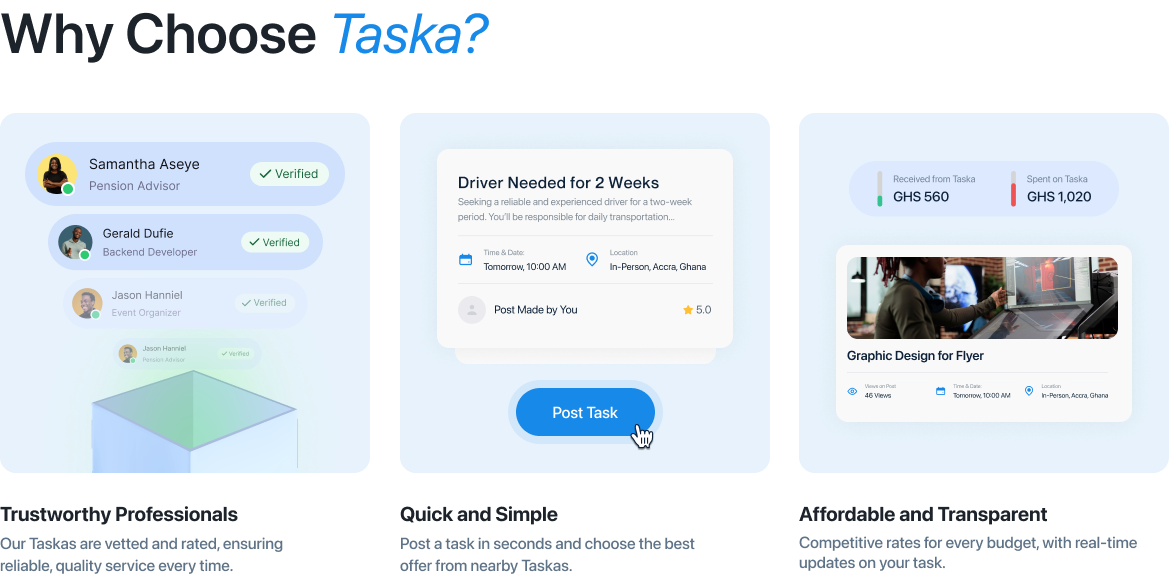
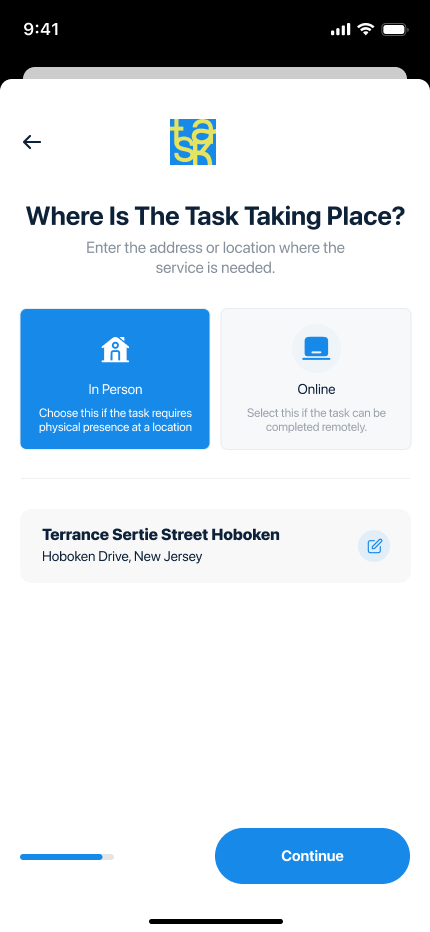
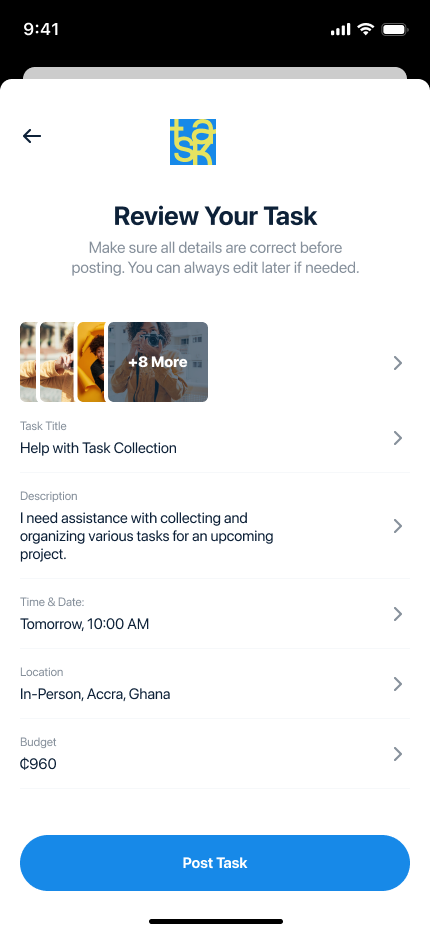
The Food and Drugs Authority (FDA) partnered with Digiits to create a modern, user-friendly website that would enhance public engagement and streamline access to critical information. The new platform was designed to address the diverse needs of the FDA’s stakeholders, including businesses, healthcare professionals, and the general public. By combining intuitive navigation, visually appealing design, and robust functionality, the redesigned website became a vital tool for improving public health awareness and regulatory compliance.
Task
Designing a Comprehensive, User-Centric Digital Platform Digiits was tasked with completely overhauling the FDA’s website to achieve the following goals: Create an intuitive, accessible interface that caters to diverse audiences, including businesses, healthcare professionals, and the general public. Streamline access to regulatory guidelines, safety alerts, and product registration information. Develop efficient, user-friendly online tools for license applications, approvals, and complaints. Ensure compliance with accessibility standards and scalability for future updates and expansions. Enhance public trust through transparency and an engaging digital experience.